The clay mineral palygorskite and the common dye known as indigo form spontaneously upon mild heating a very resistant hybrid pigment with a vivid blue color. Near-infrared spectroscopy (NIR), turned out to be the ideal tool for studying the structure of the hybrid, which is relevant to the famous meso-American pigment known as Maya blue. Palygorskite particles are lath-like. They have a chessboard cross-section of alternating aluminosilicate ribbons and hydrated channels, as well as abundant hydrophilic silanol groups on their outer surface. At the reaction temperature (up to 130 oC) both the tunnels and the surface SiOH dehydrate, thereby inducing well-defined spectral changes in the NIR, which are reversible upon cooling at ambient temperature and relative humidity. How can the binding site of indigo be identified? Why indigo, among many organics, is so unique in forming a stable complex with palygorskite at loadings up to 10 wt%? Is the formation of the complex limited to the (nearly ideal) variety found in Yucatan, Mexico? Answers to these (and other) questions can be found in Tsiantos et al., 2014, Tsiantos et al., 2012, Sanchez del Rio et al., 2009.
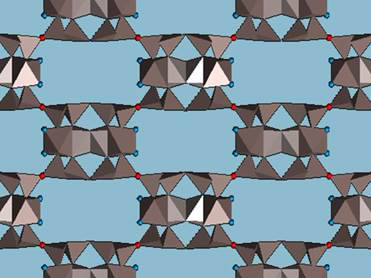

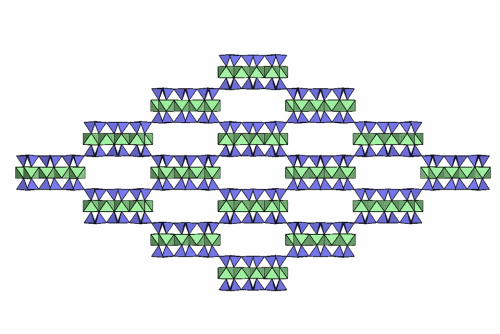 Folding sepiolite and palygorskite
Folding sepiolite and palygorskite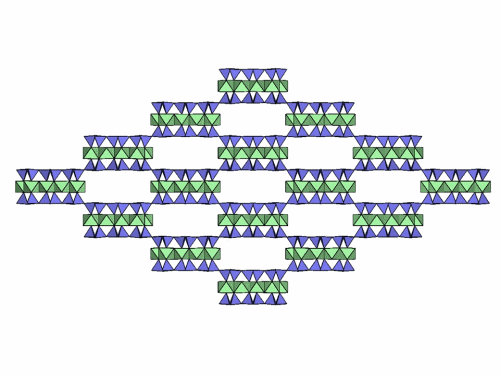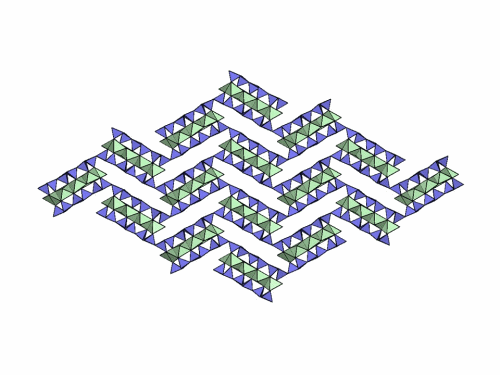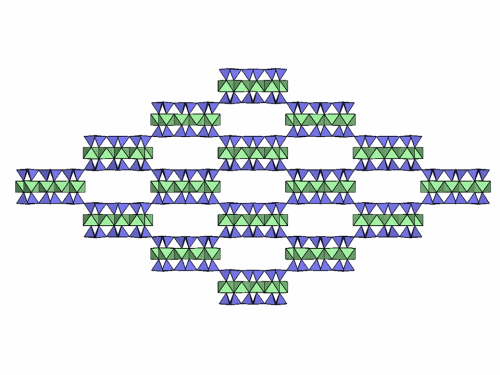
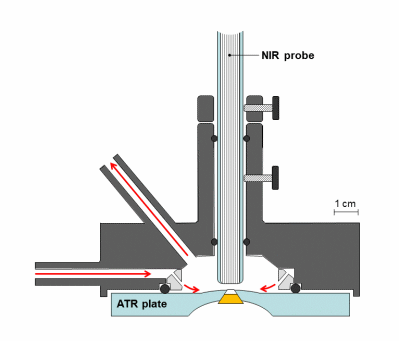 Synchronous NIR and ATR spectral acquisition
Synchronous NIR and ATR spectral acquisition 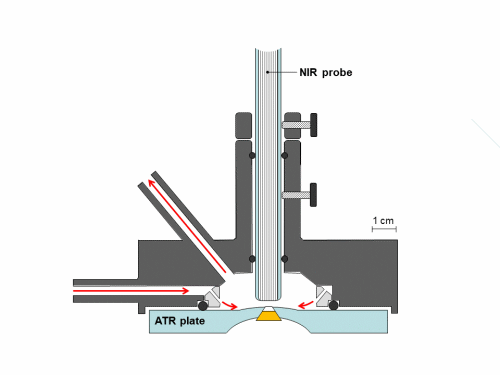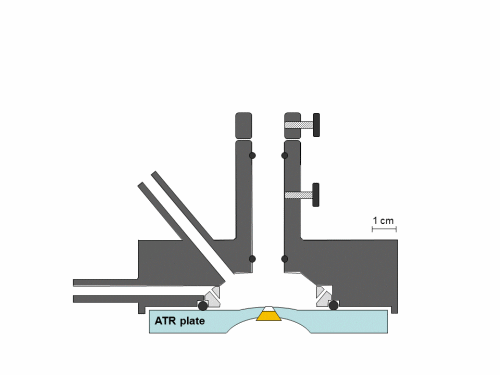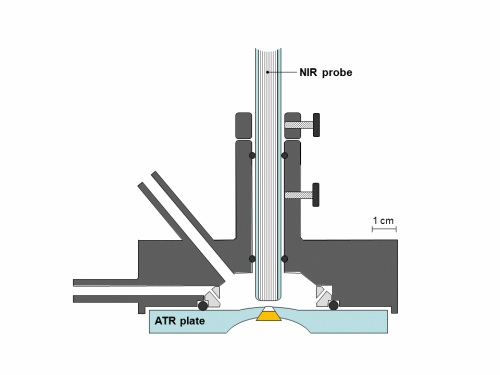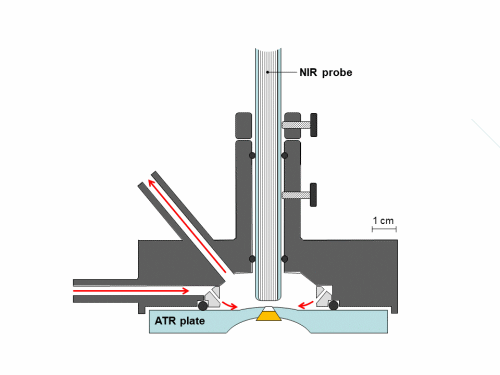
 A new method for measuring the layer charge of smectites
A new method for measuring the layer charge of smectites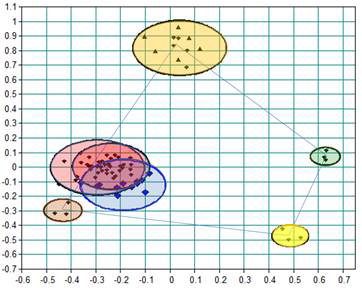 Multivariate analysis of large spectral datasets
Multivariate analysis of large spectral datasets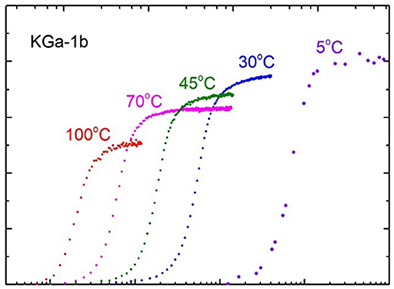 Re-interpreting the sigmoidal kinetics of intercalation in kaolinite
Re-interpreting the sigmoidal kinetics of intercalation in kaolinite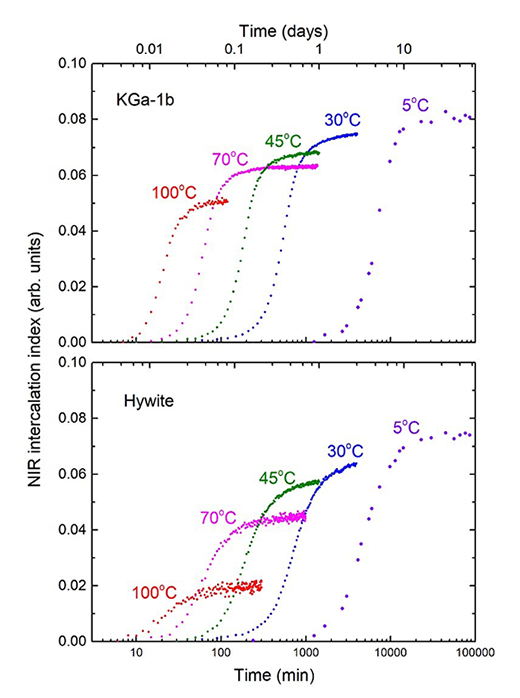
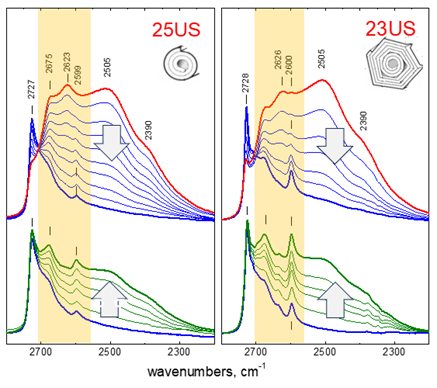 Formation and structure of halloysite nanotubes
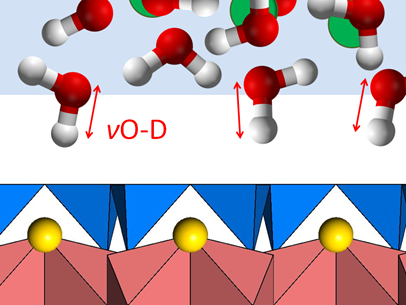
Formation and structure of halloysite nanotubes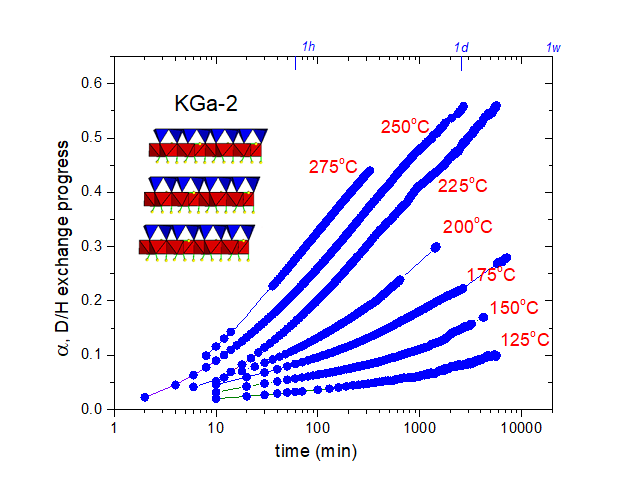 High-temperature D/H isotope exchange in kaolinite
High-temperature D/H isotope exchange in kaolinite