Olfaction plays a key role in insects, regulating essential behaviors such as i) host-seeking through the detection of organic compounds released by human, animals and plants ii) detection of species specific chemical signals such as sexual and alarm pheromones.
Mosquitoes are the primary transmitters of multiple parasites and viruses that cause malaria, dengue fever, West Nile encephalitis, microcephaly in newborn infants due to Zika virus and many other serious and often fatal diseases. Furthermore, agricultural and farm livestock insect pests, represent primary factors leading to reduced production of major crops and increased losses of farm animals.
Since 2010, Dr. Zographos’ research focuses on biochemical, molecular, functional and structural characterization of Mosquito and Pest insects Odorant Binding Proteins (OBPs), as molecular targets for the structure-based discovery of novel host-seeking disruptors, repellents or attractants. Since 2020, the research in insect olfaction was expanded to the mosquito 7-transmembrane Odorant Receptor coreceptor (ORco). The goal of this direction is the application of OBP- and ORco-structure-based approaches for the discovery of multiple new and effective agents to be employed in the effort to reduce the spread of insect-transmitted infectious diseases as well as control insects of agricultural importance.
OBP-structure-aided repellent discovery (Tsitsanou K.E., Liggri, P.G.V Stamati, E.C.V, E-Christodoulou, E-A)
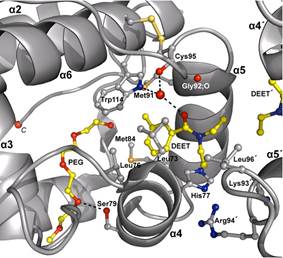 Much physiological and behavioral evidence has been provided suggesting that insect Odorant Binding Proteins (OBPs) are indispensable for odorant recognition and thus appealing targets for structure-based discovery and design of novel host-seeking disruptors.
Much physiological and behavioral evidence has been provided suggesting that insect Odorant Binding Proteins (OBPs) are indispensable for odorant recognition and thus appealing targets for structure-based discovery and design of novel host-seeking disruptors.
We have proposed OBPs as valuable molecular targets for the structure-based discovery and design of disruptors of normal olfactory and host seeking mosquito behavior (Tsitsanou et al., 2012, Tsitsanou, Drakou, et al., 2013, Drakou et al., 2017, Liggri et al., 2019). Significantly, we have now developed and successfully validated a Ligand- & OBP-structure-aided Discovery Protocol that led to identification of novel bioactive leads, attesting the applicability of the OBP-structure-aided discovery method (Thireou et al., 2018, Zographos et al., 2018). Therefore, the study on OBPs of A. gambiae and the determination of their 3D-structures and binding specificities could help us understand the molecular basis of odorant detection by OBPs, in this and other anthropophilic species, towards the development of new effective agents for the prevention of mosquito-borne diseases.
Advanced Research on the 3D Structure of the Mosquito Odorant Receptor Coreceptor (Tsitsanou K.E., Liggri, P.G.V)
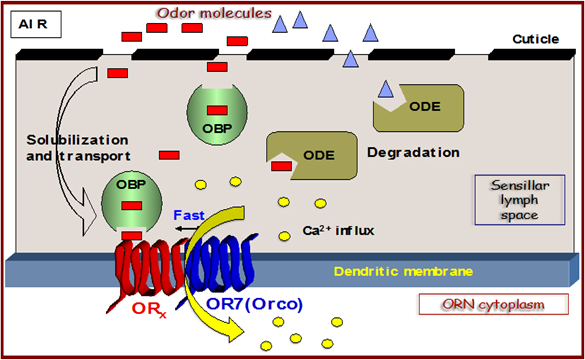
Insect Odorant Receptors (ORs) play the central role in the manifestation of behavioral responses. In insects, including mosquitoes, each Olfactory Receptor Neuron (ORN) expresses a divergent member of the OR family (ORx) and a highly conserved one, Odorant Receptor coreceptor (ORco), which is abundantly expressed in insect ORNs. Formation of ORx-ORco heteromers leads to functional ligand (odor)-gated non-selective cation channels, directly activated by odors or pheromone ligands. Recent findings have shown that ORco acts as a ligand-gated channel in the absence of ORx and that it possesses an allosteric site to which modulators can bind directly and noncompetitively inhibit OR activation by odorants (Antagonists) or act as positive allosteric modulators (Agonists) of heteromeric channel function (Iatrou et al., 2022).
The project aims at the biochemical, molecular, functional and structural characterization of the 7-transmembrane Odorant Receptor coreceptor (ORco) of the mosquito Anopheles gambiae, applying the most up-to-date methodology and cutting-edge technology, including Nanodisk technology, Robotic high-throughput in meso crystallogenesis, Micro- and serial-crystallography using synchrotron radiation and high resolution cryo-Electron Microscopy. The results to be obtained from the successful implementation of this project, in addition to being of great importance to public health, could be further exploited for the development of ecologically-smart methods for the control of agricultural and farm livestock insect pests. Therefore, this project could have a major economic impact on the agricultural and livestock raising sectors.


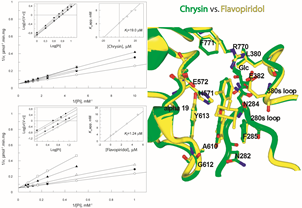 Glycogen phosphorylase (GP; EC 2.4.1.1) is a key enzyme in glycogen metabolism that catalyzes the first step in the degradation of glycogen to yield glucose 1-phosphate (glucose-1-P). As such, GP has a pivotal role in human carbohydrate catabolism by initiating the enzyme cascade that releases glucose from glycogen deposits to serve the energy needs of the organism. Because of the central role of GP in glucose homeostasis, the enzyme has been investigated for therapeutic intervention in type 2 diabetes and validated as a molecular target for the discovery of novel antidiabetic drugs that will inhibit hepatic glucose production. Over the past decade, X-ray crystallographic, enzyme kinetic and structure-assisted inhibitor design studies, have led to the discovery of more than 100 inhibitors of glycogen phosphorylase (GP) enzyme (target for type 2 diabetes mellitus), including new synthetic inhibitor classes (Leonidas et al., 2021, Kyriakis et al., 2020, Szabo et al., 2019, Fischer et al., 2019, Bokor et al., 2017, Parmenopoulou et al., 2014, Czifrak et al., 2014, Kantsadi, Hayes, et al., 2012, Parmenopoulou et al., 2012, Kantsadi, Manta, et al., 2012, Manta et al., 2012, Nagy et al., 2012) and natural products such as flavonoids and terpenes (Tsitsanou, Hayes, et al., 2013). The essential binding properties of these compounds are analyzed in an effort to provide rationalizations for the affinities of these compounds, carry on a more detailed SAR study and to exploit the molecular interactions that might give rise to better inhibitors, potential agents for controlling hyperglycaemia in T2D.
Glycogen phosphorylase (GP; EC 2.4.1.1) is a key enzyme in glycogen metabolism that catalyzes the first step in the degradation of glycogen to yield glucose 1-phosphate (glucose-1-P). As such, GP has a pivotal role in human carbohydrate catabolism by initiating the enzyme cascade that releases glucose from glycogen deposits to serve the energy needs of the organism. Because of the central role of GP in glucose homeostasis, the enzyme has been investigated for therapeutic intervention in type 2 diabetes and validated as a molecular target for the discovery of novel antidiabetic drugs that will inhibit hepatic glucose production. Over the past decade, X-ray crystallographic, enzyme kinetic and structure-assisted inhibitor design studies, have led to the discovery of more than 100 inhibitors of glycogen phosphorylase (GP) enzyme (target for type 2 diabetes mellitus), including new synthetic inhibitor classes (Leonidas et al., 2021, Kyriakis et al., 2020, Szabo et al., 2019, Fischer et al., 2019, Bokor et al., 2017, Parmenopoulou et al., 2014, Czifrak et al., 2014, Kantsadi, Hayes, et al., 2012, Parmenopoulou et al., 2012, Kantsadi, Manta, et al., 2012, Manta et al., 2012, Nagy et al., 2012) and natural products such as flavonoids and terpenes (Tsitsanou, Hayes, et al., 2013). The essential binding properties of these compounds are analyzed in an effort to provide rationalizations for the affinities of these compounds, carry on a more detailed SAR study and to exploit the molecular interactions that might give rise to better inhibitors, potential agents for controlling hyperglycaemia in T2D. The RAS-RAF-MEK-ERK signal transduction cascade is arguably the most important oncogenic pathway in human cancers. A-RAF, B-RAF, and C-RAF belonging to the RAF family of protein serine/threonine kinases are critical effectors of this pathway. B-RAF mutations occur in a variety of cancers (30-60% of melanomas, 30-50% of thyroid cancers, and 5-20% of colorectal cancers). Therefore, B-RAF kinase has been recognized as a key target for cancer treatment. The most common activating BRAF point mutation accounting for 90% of all B-RAF mutations results in a substitution of glutamic acid (E) for valine (V) at position 600 of the aminoacid sequence (B-RAFV600E), leading to a mutant oncogenic version of the RAF kinase enzyme. B-
The RAS-RAF-MEK-ERK signal transduction cascade is arguably the most important oncogenic pathway in human cancers. A-RAF, B-RAF, and C-RAF belonging to the RAF family of protein serine/threonine kinases are critical effectors of this pathway. B-RAF mutations occur in a variety of cancers (30-60% of melanomas, 30-50% of thyroid cancers, and 5-20% of colorectal cancers). Therefore, B-RAF kinase has been recognized as a key target for cancer treatment. The most common activating BRAF point mutation accounting for 90% of all B-RAF mutations results in a substitution of glutamic acid (E) for valine (V) at position 600 of the aminoacid sequence (B-RAFV600E), leading to a mutant oncogenic version of the RAF kinase enzyme. B-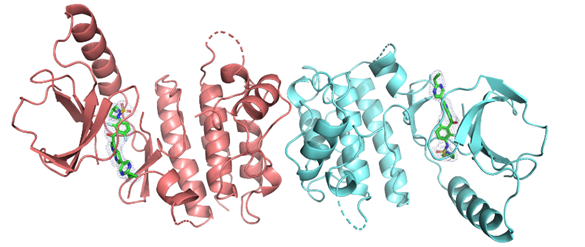
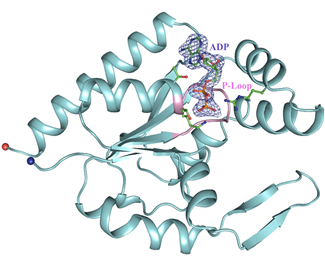 human Coilin Interacting Nuclear ATPase Protein (hCINAP) directly interacts with coilin, a marker protein of Cajal Bodies (CBs), nuclear organelles involved in the maturation of small nuclear ribonucleoproteins (Santama et al, 2005). hCINAP has previously been designated as an adenylate kinase (AK6), but it exhibits structural features characteristic of ATPase/GTPase proteins (Walker motifs A and B) and also intrinsic ATPase activity. In 2012 we reported the first high-resolution structure of hCINAP in complex with the substrate ADP and the structure of the ternary complex hCINAP-Mg2+ADP-PO43-. Structural analysis suggested a functional role for His79 in the Walker B motif. We have shown that in vivo expression of hCINAP-H79G in human cells is toxic and drastically deregulates the number and appearance of CBs. Our findings suggest that hCINAP may not simply regulate nucleotide homeostasis, but may have broader functionality, including control of CBs assembly (Drakou et al, 2012). In last decade hCINAP has been reported to be an important regulator of Ribosomal Protein-HDM2-p53 pathway and a potential anticancer drug target. The detailed understanding of hCINAP function and its interaction with ligands and protein partners will give new insights into basic steps in eukaryotic gene expression, such as regulation of RNA processing, and assess its potential as a novel pharmacological target to combat cancer.
human Coilin Interacting Nuclear ATPase Protein (hCINAP) directly interacts with coilin, a marker protein of Cajal Bodies (CBs), nuclear organelles involved in the maturation of small nuclear ribonucleoproteins (Santama et al, 2005). hCINAP has previously been designated as an adenylate kinase (AK6), but it exhibits structural features characteristic of ATPase/GTPase proteins (Walker motifs A and B) and also intrinsic ATPase activity. In 2012 we reported the first high-resolution structure of hCINAP in complex with the substrate ADP and the structure of the ternary complex hCINAP-Mg2+ADP-PO43-. Structural analysis suggested a functional role for His79 in the Walker B motif. We have shown that in vivo expression of hCINAP-H79G in human cells is toxic and drastically deregulates the number and appearance of CBs. Our findings suggest that hCINAP may not simply regulate nucleotide homeostasis, but may have broader functionality, including control of CBs assembly (Drakou et al, 2012). In last decade hCINAP has been reported to be an important regulator of Ribosomal Protein-HDM2-p53 pathway and a potential anticancer drug target. The detailed understanding of hCINAP function and its interaction with ligands and protein partners will give new insights into basic steps in eukaryotic gene expression, such as regulation of RNA processing, and assess its potential as a novel pharmacological target to combat cancer.