For years, the foundations of cancer treatment were surgery, chemotherapy, and radiation therapy. Over the last two decades, targeted therapies like imatinib (Gleevec®) and trastuzumab (Herceptin®)—drugs that target cancer cells by homing in on specific molecular changes seen primarily in those cells—have also cemented themselves as standard treatments for many cancers. But over the past several years, immunotherapy—therapies that enlist and strengthen the power of a patient’s immune system to attack tumors—has emerged as what many in the cancer community now call the “fifth pillar” of cancer treatment.
One of the most successful attempts in the field of immunotherapy is T Cell Transfer therapy, which is gaining a lot of attention lately due to the indisputable success of Chimeric Antigen Receptor T Cell (CAR-T cells) therapy in B cell lymphomas and Acute Lymphoblastic Leukemia (ALL), in which over 80% of the patients showed complete regression of the disease.
Likewise, Adoptive T cell therapy using ex vivo expanded TILs has also led to complete regression of 20-25 % of patients with metastatic melanoma and other solid tumors. Such remarkable effects were mediated by T cells that specifically target mutant peptides (neoantigens) encoded by genes sharing de novo somatic mutations. Adoptive T cell therapy clinical trials have revolutionized the field of cancer immunotherapy, which now has additional prominent allies, such as monoclonal antibodies against PD1/PDL1 and CTLA 4 that apparently act on the activation of T cells, which recognize and attack cancer cells.
The therapeutic potential of the ACT, which is based on tumor-specific lymphocytes, was first tested by Fefer and Rosenberg about 40 years ago showing only limited efficacy. In recent years, due to the advancement of cellular and molecular biology techniques, such as T cell extraction, expansion and activation ex vivo, and genetic engineering techniques, ACT is becoming a viable therapeutic option for cancer patients who are refractory to conventional therapy. The ACT can also include dendritic cells (DCs) and/or immune effector cells, or combination of both. DCs are often used as vaccine carriers or antigen presenting cells (APCs) to prime naive T cells in vitro or in vivo. Cytotoxic T lymphocytes (CTL) and natural killer cells (NK) are the major effector cells in naturally occurring anti-tumor response and were exploited very early as tool cells for the ACT. An impressive success of ACT has been achieved in several types of cancers during the last two decades, and the most prominent progress was made in B lymphocyte leukemia and lymphoma. Since then, promising results were obtained in a variety of solid tumors, including metastatic melanoma, renal cell carcinoma (RCC), nasopharyngeal cancer, gastric cancer, hepatocellular carcinoma, and lung cancer. ACT with TILs is yet the most effective immunotherapy strategy for patients with metastatic melanoma.
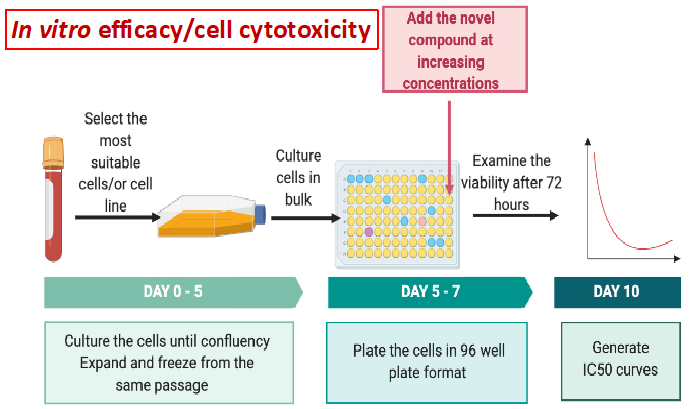
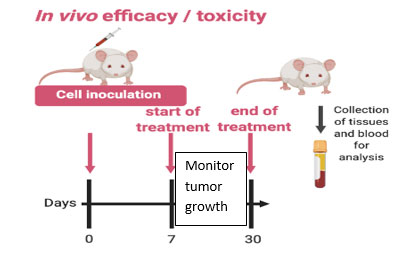
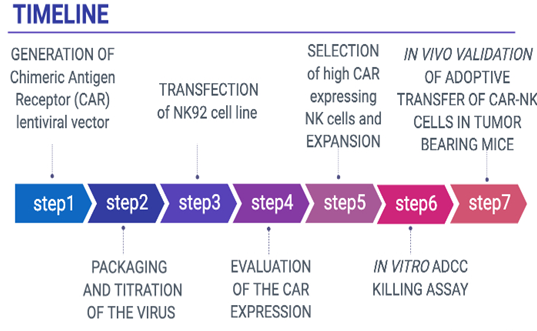
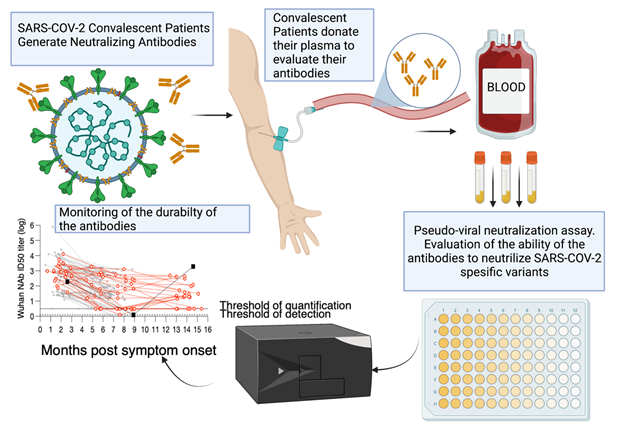
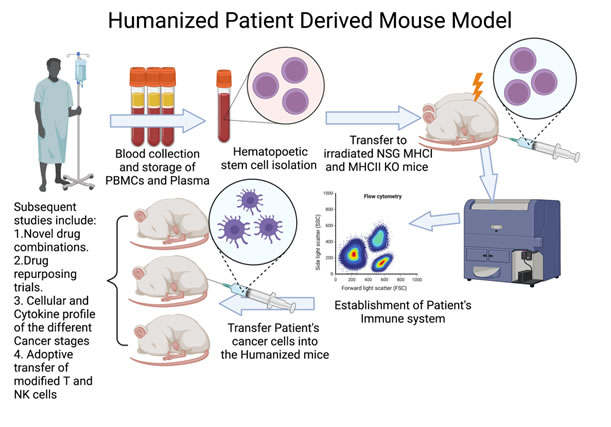
The team will focus on i) identifying and characterizing CD8+ Tumor Infiltrating T Lymphocytes, which have the ability to recognize specific somatic mutations in cancer cells and ii) evaluate the in vivo efficacy of these T cells alone or in combination with immune checkpoint inhibitors. The fresh tumor samples will be provided with patients’ consent under the already existing collaboration between NHRF and the General Hospital of Athens, under the consortium ‘’Medical precision Unit’’. The proposed work plan is depicted below.
Mission
Our goal is to provide useful information to the physicians regarding the mutational landscape of the tumor, and the underlying response of the immune system to CANCER NEO-ANTIGENS trying to modify the therapeutic approach towards a personalized therapy, using our in vitro and in vivo data. With the hope that our work will be one of the first attempts to show the lifesaving benefits of ACT and eventually engage our legislative system to incorporate in the near future adoptive cell transfer of modified ex vivo cells to the clinic to completely eradicate some cancer types and expand life expectancy for cancer patients.
Selected Publications
- Stochastic phenotype switching leads to intratumor heterogeneity in human liver cancer, Matak, A., Lahiri, P., Ford, E., Pabst, D., Kashofer, K., Stellas, D., Thanos, D., and Zatloukal, K., Hepatology 68(3):933-948, 2018
- Heterodimeric interleukin 15 (hetIL-15) treatment decreases primary breast cancer 4T1 tumors and alleviates the metastatic burden, Stellas, D., Dimas, K., Nagy, B.A., Valentin, A., Felber, B.K., and Pavlakis, G.N., In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; 2018 Apr 14-18; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2018
- Stellas, D., Karaliota, S., Stravokefalou, V., Nagy, B.A., Felber, B.K., and Pavlakis, G.N., American Association for Cancer Research Annual Meeting, Heterodimeric IL-15 monotherapy results in complete regression of EO771 murine breast tumors through cDC1-lymphocyte interactions and induction of antitumor immunity. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 March 29-3 Apr, Atlanta. Georgia (GA): AACR; Cancer Res 2019
- Watson, D.C., Yung, B.C., Bergamaschi, C., Chowdhury, B., Bear, J., Stellas, D., Morales-Kastresana, A., Jones, J.C., Felber, B.K., Chen, X., and Pavlakis, G.N.: Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15 / Lactadherin complexes. J Extracell Vesicles. 28;7(1):1442088. 2018
- Bergamaschi,C., Pandit, H., Nagy,B., Stellas, D., Jensen, S., Bear,J., Cam,M., Valentin,A., Fox,B., Felber, BK., Pavlakis, GN. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J Immunother Cancer. 2020 May;8(1):e000599. doi: 10.1136/jitc-2020-000599