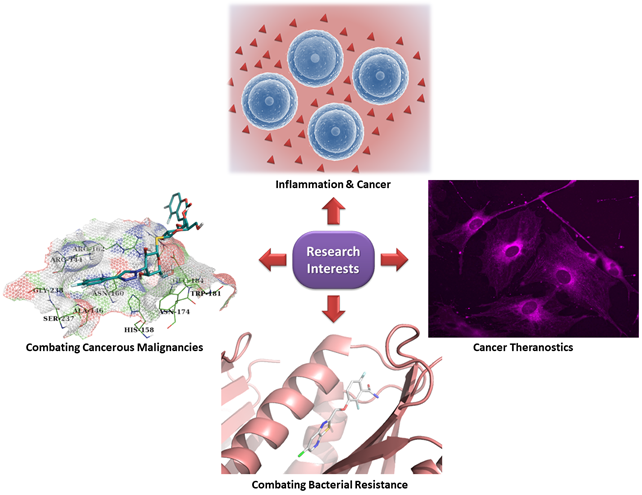
Cancer as a condition refers in abnormal and uncontrolled cell growth across any organ or tissue of the body, remaining still unaddressed until today with treatment issues like general toxicity, side effects, relapse and emerging drug resistance. Appearance and progression of the disease are based on complex and multifactorial etiologies. Among the most important proteins recognized and implicated in numerous cancer hallmarks & inflammatory processes are Galectins. This family of proteins showcases cancer specific functions and their abundant expression in such malignant conditions makes them appealing targets for inhibition or modulation giving space for the development of novel cancer treatments.
 |

 The K. C. Prousis group is focused on activities combining the fields of Synthetic Medicinal Chemistry and Chemical Biology. The group aims to provide new therapeutic approaches against several lethal diseases with high socioeconomic impact including Cancer, Inflammation, Antibacterials, Neurodegenerative Diseases and Parasitic Neglected Diseases.
The K. C. Prousis group is focused on activities combining the fields of Synthetic Medicinal Chemistry and Chemical Biology. The group aims to provide new therapeutic approaches against several lethal diseases with high socioeconomic impact including Cancer, Inflammation, Antibacterials, Neurodegenerative Diseases and Parasitic Neglected Diseases.