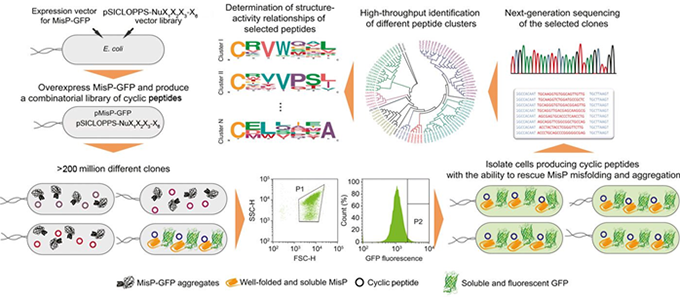
It has been widely recognized that many incurable diseases with an enormous socioeconomic impact, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, type 2 diabetes etc., are initiated by a common molecular mechanism: the misfolding of specific proteins. With the ultimate goal of identifying compounds with potentially therapeutic effects against these disorders, we have developed a highly versatile synthetic biology platform for discovering macrocyclic rescuers of disease-associated protein misfolding. In this system, large combinatorial libraries of cyclic oligopeptides (>200 million members) are biosynthesized in Escherichia coli cells and simultaneously screened for their ability to rescue disease-associated protein misfolding using an ultrahigh-throughput genetic screen. Hit compounds identified by this screen, are subjected to more detailed evaluation by biochemical and biophysical methods of protein analysis, and their ability to inhibit protein misfolding-induced pathogenicity is evaluated using appropriate mammalian cell assays and in vivo models of the disease of interest. Medicinal chemistry approaches are subsequently employed to optimize the biological properties of the bioactive macrocycles in order to generate therapeutic leads against the target diseases.
Schematic representation of our engineered bacterial platform for the discovery of macrocyclic rescuers of MisP misfolding and aggregation. MisP: misfolding-prone protein; pMisP-GFP: plasmid encoding a MisP-GFP fusion; pSICLOPPS-NuX1X2X3-X6: vector library encoding the combinatorial library of cyclic tetra-, penta-, hexa and heptapeptides P: sorting gate.
We are currently targeting the following proteins involved in misfolding diseases:
Amyloid-β peptide & Alzheimer’s disease
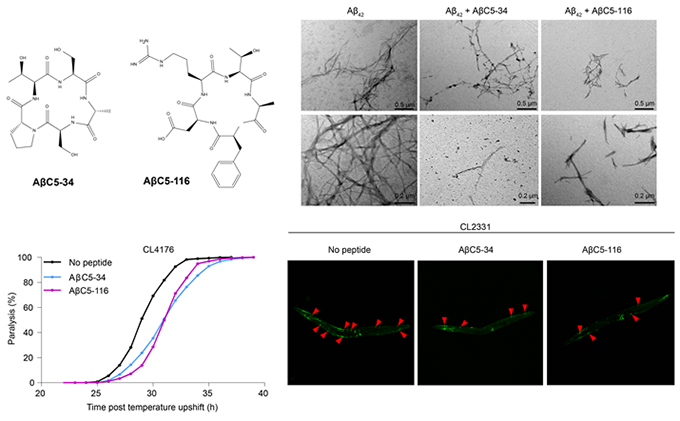
(top, left) Chemical structures of the selected cyclic pentapeptides ΑβC5-34 and ΑβC5-116; (top, right) TEM images of aged Αβ42 solutions in the presence and absence of the selected cyclic pentapeptides ΑβC5-34 and ΑβC5-116; (bottom, left) Paralysis of C. elegans worms expressing human Αβ in their body wall muscles under the control of a heat-inducible promoter, in the absence and presence of the selected cyclic oligopeptides ΑβC5-34 and ΑβC5-116; (bottom, right) Fluorescence microscopy images of C. elegans nematodes showing reduced formation of Αβ aggregates when treated with two of our selected cyclic pentapeptides ΑβC5-34 and ΑβC5-116. Taken from Matis, I. et al., Nature Biomedical Engineering, 2017.
Cu/Zn superoxide dismutase (SOD1) & Amyotrophic lateral sclerosis (ALS)
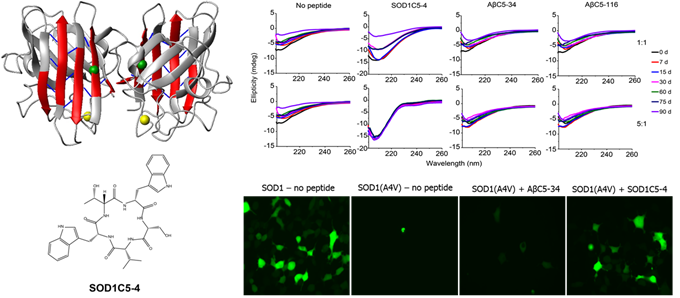
(top, left) Structure of homodimeric SOD1 (taken from Banci, L. et al., Proc Natl Acad Sci USA, 2009); (bottom, left) Chemical structure of the selected cyclic pentapeptide SOD1C5-4; (top, right) Circular dichroism spectra of SOD1(A4V) incubated with/without the selected cyclic pentapeptides SOD1C5-4, ΑβC5-34 or ΑβC5-116 at room temperature for 90 d (1:1 and 5:1 indicate cyclic peptide:SOD1(A4V) molar ratios). Taken from Matis, I. et al., Nature Biomedical Engineering, 2017; (bottom, right) ΗΕΚ293 cells transiently expressing SOD1-GFP or SOD1(A4V)-GFP in the absence and presence of the selected peptide SOD1C5-4 or the Αβ-targeting cyclic pentapeptide ΑβC5-34, and visualized by confocal microscopy.
p53 & Cancer
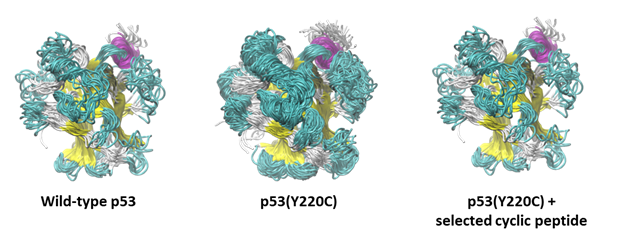
Conformational stabilization of the DNA-binding (core) domain of the carcinogenic, misfolding-prone p53 variant Y220C by a selected cyclic oligopeptide as revealed by molecular dynamics simulations.
Polyglutaminated (polyQ) huntingtin & Huntington’s disease
α-Synuclein & Parkinson’s disease
Immunoglobulin light chains & Light chain amyloidosis
β2-Microglobulin (β2m) & Dialysis-related amyloidosis
Rhodopsin & Retinitis pigmentosa
Highlights
- We have identified hundreds of head-to-tail cyclic oligopeptide sequences that inhibit the misfolding and aggregation of Αβ. For four of them, termed ΑβC5-34, ΑβC5-116, ΑβC7-1 and ΑβC7-14 we have shown that they interfere with the normal course of Aβ aggregation and the formation of typical Aβ fibrils, generating species with reduced binding to the neuronal surface and reduced cytotoxicity in vitro and in vivo (Matis, I. et al., Nature Biomedical Engineering, 2017; Delivoria, D.C. et al., Sciences Advances, 2019).
- We have identified hundreds of head-to-tail cyclic oligopeptide sequences that inhibit the misfolding and aggregation of mutant human SOD1, associated with familial forms of ALS. For one of them, termed SOD1C5-4, we have shown that it interferes with the normal course of mutant SOD1 aggregation and the formation of typical mutant SOD1 aggregates in vitro. We have found that SOD1C5-4 is cell-permeable and that it reduces the formation and cytotoxicity of mutant SOD1 aggregates in human cell lines (Matis, I. et al., Nature Biomedical Engineering, 2017).



 Dr. Georgios Skretas, was awarded a Consolidator Grant from the European Research Council (ERC) to develop engineered bacteria that accelerate drug discovery against diseases caused by protein misfolding. [
Dr. Georgios Skretas, was awarded a Consolidator Grant from the European Research Council (ERC) to develop engineered bacteria that accelerate drug discovery against diseases caused by protein misfolding. [