Although the research towards the development of small molecules as proteasome activators is scarce, the results so far suggest that activation of the proteasome complex constitutes a pioneer strategy for the deceleration of ageing and age-related diseases A novel class of hybrid compounds that combine structural features of benzothiophene and of hydroxytyrosol connected through carefully selected linkers, was designed and synthesized by our group, leading to the development of an optimum structural proteasome activator.
STHENOS-b 2017-2020 in collaboration with members of other research groups of I.C.B.
This class of compounds was further exploited by synthesizing more analogues. The biological results to date show great promise with regards to the anti-ageing and neuroprotective effects of the new analogues in cellulo and in organismal level.
RESET: “Bio-inspired antiaging proteasome activators” In collaboration with Intermed SA.


 Recently from our laboratory we reported the design,
synthesis, and preliminary biological data of two novel endocannabinoid
molecular probes referred to as "(13S)-methyl substituted
anandamide analogs". It is especially worthy of note that (13S)-methyl
substituted anandamide is among the endocannabinoid analogs with the
highest CB1-binding affinity known to date.
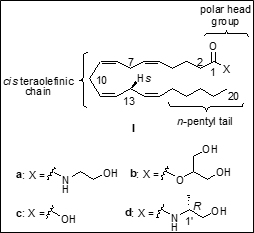
Recently from our laboratory we reported the design,
synthesis, and preliminary biological data of two novel endocannabinoid
molecular probes referred to as "(13S)-methyl substituted
anandamide analogs". It is especially worthy of note that (13S)-methyl
substituted anandamide is among the endocannabinoid analogs with the
highest CB1-binding affinity known to date. 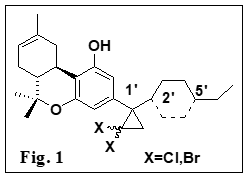 Our laboratory has pioneered in the design
and use of “cannabinoid ligands” as tools for studying the binding domain of
the different classes of ligands within the CB1 and CB2 receptors. Earlier
work from our laboratory has revealed a strong correlation between
the presence of substituents at C1¢-position of (-)-D8-tetrahydrocannabinols
((-)-D8-THCs,) and the existence of a subsite
within the CB1 and CB2 cannabinoid receptor binding domain.
Our laboratory has pioneered in the design
and use of “cannabinoid ligands” as tools for studying the binding domain of
the different classes of ligands within the CB1 and CB2 receptors. Earlier
work from our laboratory has revealed a strong correlation between
the presence of substituents at C1¢-position of (-)-D8-tetrahydrocannabinols
((-)-D8-THCs,) and the existence of a subsite
within the CB1 and CB2 cannabinoid receptor binding domain.