Transition-metal homogeneous catalysis
Our investigations include the development of transition-metal (e.g. Ru, Rh, Pd, Pt) complexes with novel ligands and their evaluation as catalysts in reactions of enormous academic and industrial interest, such as hydroformylation, hydrogenation, coupling reactions (Heck, Suzuki).
More specifically, our research activities include:
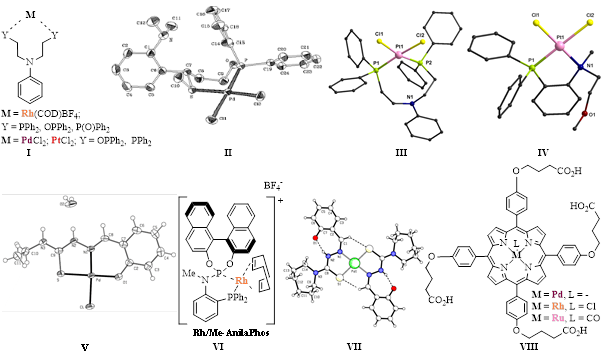
(a) Catalysis by hybrid and hemilabile phosphorus ligands (e.g. phosphines, phosphine oxides, phosphinites, mixed phosphine-phosphinites) possessing additional potential donors such as oxygen, nitrogen, sulfur (e.g. I – IV).
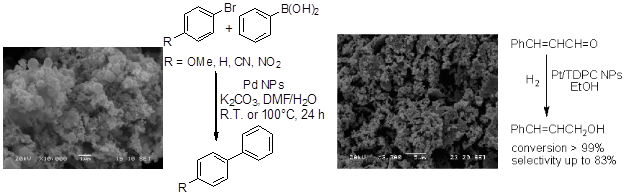
(b) Catalysis in air by phosphane-free ligands such as thiosemicarbazones (e.g. V, VII) and chalcogen-containing Schiff bases. We are pioneers in the use of thiosemicarbazones as catalyst precursors for palladium-catalyzed coupling reactions (Heck, Suzuki), under aerobic conditions, and one of our Pd complexes is commercially available from MERCK (SIGMA-ALDRICH) (Product No.: 674125) and other companies.
(c) Asymmetric catalysis with new chiral amino diphosphite, phosphonite, phosphite-phosphoramidite and phosphine-phosphoramidite ligands, such as Me-AnilaPhos (VI) as a highly efficient ligand for the rhodium-catalyzed enantioselective olefin hydrogenation (100% conversion after 10 min at r.t. and 1 bar pressure, 98% ee).
(d) Catalysis by high energy techniques such as microwave irradiation. This technique was used to the Suzuki coupling in air, catalyzed by a palladium complex with a thiosemicarbazone ligand (VII), which was totally inactive under conventional heating.
(e) Aqueous catalysis offering environmental benefits and also the advantage of recycling and reusing the catalyst. We have published the first study concerning the evaluation of air-stable and water-soluble metalloporphyrins (VIII) in the Suzuki reaction and also the selective hydrogenation of unsaturated aldehydes in neat water or in an aqueous/organic biphasic system. The catalysts could be easily recycled and reused.
Selected Publications
- I.D.
Kostas*, B.R. Steele* Catalysts 2020, 10 (10),
1107, 1–40
“Thiosemicarbazone Complexes of Transition Metals as Catalysts for
Cross-Coupling Reactions”.
https://doi.org/10.3390/catal10101107 - P.-C. Ioannou, C. Arbez-Gindre, M. Zoumpanioti, C. P.
Raptopoulou, V. Psycharis, I. D. Kostas*, P. Kyritsis* J.
Organomet. Chem. 2019, 879, 40–46 “Catalytic
reactivity of the complexes [Pd{(Ph2P)2N(tBu)-P,P´}X2],
X = Cl, Br, I, in
the Suzuki-Miyaura C−C coupling reaction: Probing effects of the halogeno
ligand X- and the ligand’s tBu group”.
https://doi.org/10.1016/j.jorganchem.2018.10.006 - I.K. Stamatopoulos, M. Kapsi, M. Roulia, G.C. Vougioukalakis,
C.P. Raptopoulou, V. Psycharis, I.D. Kostas*, L. Kollár*, P. Kyritsis* Polyhedron 2018, 151, 292–298 “Structural features and catalytic reactivity
of [Pd{(Ph2P)2N(CH2)3Si(OCH3)3-κP,P’}I2]
and related complexes in hydroalkoxycarbonylation and Suzuki-Miyaura C–C
cross-coupling reactions”.
https://doi.org/10.1016/j.poly.2018.05.041 - I.D. Kostas*, G. Antonopoulou, C.
Potamitis, C.P. Raptopoulou, V. Psycharis J. Organomet. Chem. 2017, 828, 133-141 “Platinum Complexes with a
methoxy-amino phosphine or a nitrogen-containing bis(phosphine) ligand.
Synthesis, characterization and application to hydrogenation of trans-cinnamaldehyde”.
https://doi.org/10.1016/j.jorganchem.2016.11.036 - I.D.
Kostas*,
A.-C. Tenchiu, C. Arbez-Gindre, V. Psycharis, C.P. Raptopoulou Catal. Commun. 2014, 51, 15-18 “Room-temperature Suzuki-Miyaura coupling of aryl bromides
with phenylboronic acid catalyzed by a palladium complex with an inexpensive
nitrogen-containing bis(phosphinite) ligand”.
https://doi.org/10.1016/j.catcom.2014.03.014 - K.A.
Vallianatou, D.J. Frank, G. Antonopoulou, S. Georgakopoulos, E. Siapi, M.
Zervou, I.D. Kostas* Tetrahedron Lett. 2013, 54(5),
397-401 “Rhodium-catalyzed asymmetric olefin hydrogenation by easily accessible
aniline- and pyridine-derived chiral phosphites”.
https://doi.org/10.1016/j.tetlet.2012.11.023 - C.
Stangel, G. Charalambidis, V. Varda, A.G. Coutsolelos,* I.D. Kostas* Eur.
J. Inorg. Chem. 2011, (30), 4709-4716
“Aqueous–Organic Biphasic Hydrogenation of trans-Cinnamaldehyde Catalyzed by
Rhodium and Ruthenium Phosphane-Free Porphyrin Complexes”.
https://doi.org/10.1002/ejic.201100668 - I.D.
Kostas* Curr. Org. Synth. 2008, 5(3), 227-249 “Recent Advances on
P,N-Containing Ligands for Transition-Metal Homogeneous Catalysis”.
https://doi.org/10.2174/157017908785133447 - I.D.
Kostas*,
A.G. Coutsolelos*, G. Charalambidis, A. Skondra Tetrahedron Lett. 2007, 48(38), 6688-6691 “The first use of porphyrins as catalysts in
cross-coupling reactions: a water-soluble palladium complex with a porphyrin
ligand as an efficient catalyst precursor or the Suzuki–Miyaura reaction in
aqueous media under aerobic conditions”.
https://doi.org/10.1016/j.tetlet.2007.07.141 - K.A. Vallianatou, I.D.
Kostas*, J. Holz, A. Börner Tetrahedron Lett. 2006, 47(45),
7947-7950 “Me-AnilaPhos: A new chiral phosphine–phosphoramidite ligand for a
highly efficient Rh-catalyzed asymmetric olefin hydrogenation”.
https://doi.org/10.1016/j.tetlet.2006.08.136 - I.D. Kostas*, B.R. Steele*, A.
Terzis, S.V. Amosova, A.V. Martynov, N.A. Makhaeva Eur. J.
Inorg. Chem. 2006, (13), 2642-2646 “New Palladium
Complexes with S- or Se-Containing Schiff-Base Ligands as Efficient Catalysts
for the Suzuki-Miyaura Cross-Coupling Reaction of Aryl Bromides with
Phenylboronic Acid under Aerobic Conditions”.
https://doi.org/10.1002/ejic.200600180 - I.D.
Kostas*,
G.A. Heropoulos*, D. Kovala-Demertzi*, P.N. Yadav, J.P. Jasinski, M.A.
Demertzis, F.J. Andreadaki, G. Vo-Thanh, A. Petit, A. Loupy Tetrahedron
Lett. 2006, 47(26), 4403-4407 “Microwave-promoted
Suzuki-Miyaura cross-coupling of aryl halides with phenylboronic acid under
aerobic conditions catalyzed by a new palladium complex with a
thiosemicarbazone ligand”.
https://doi.org/10.1016/j.tetlet.2006.04.088 - I.D.
Kostas*,
F.J. Andreadaki, D. Kovala-Demertzi*, C. Prentjas, M.A. Demertzis Tetrahedron
Lett. 2005, 46(12), 1967-1970 “Suzuki-Miyaura cross-coupling
reaction of aryl bromides and chlorides with phenylboronic acid under aerobic
conditions catalyzed by palladium complexes with thiosemicarbazone ligands”.
https://doi.org/10.1016/j.tetlet.2005.02.003 - D.
Kovala-Demertzi*, P.N. Yadav, M.A. Demertzis, J.P. Jasiski, F.J. Andreadaki, I.D.
Kostas* Tetrahedron Lett. 2004, 45(14), 2923-2926
“First use of a palladium complex with a thiosemicarbazone ligand as catalyst
precursor for the Heck reaction”.
https://doi.org/10.1016/j.tetlet.2004.02.062 - I.D.
Kostas* J. Organomet. Chem. 2001, 626(1-2), 221-226 “Synthesis of
new rhodium complexes with a hemilabile nitrogen-containing bis(phosphinite) or
bis(phosphine) ligand. Application to hydroformylation of styrene”.
https://doi.org/10.1016/S0022-328X(01)00701-X