Neglected tropical diseases (NTD) caused by protozoa cause significant morbidity and mortality in the developing world.  Every year, according to the WHO, more than one million people die from complications from neglected diseases, such as African trypanosomiasis (HAT), leishmaniasis and Chagas disease (CD). Even though NTDs account for 11% of the global disease burden, of the 850 new therapeutic products approved between 2000 and 2011, only 4% (and only 1% of all approved new chemical entities (NCEs)) were indicated for neglected diseases. In line with the “One Health” concept it would be of high interest to break the chain between the parasite reservoir in animals and infections in humans reversing the economic losses and sustaining the social well-being in Europe and endemic countries. Thus, new drugs for neglected diseases are still urgently needed.
Every year, according to the WHO, more than one million people die from complications from neglected diseases, such as African trypanosomiasis (HAT), leishmaniasis and Chagas disease (CD). Even though NTDs account for 11% of the global disease burden, of the 850 new therapeutic products approved between 2000 and 2011, only 4% (and only 1% of all approved new chemical entities (NCEs)) were indicated for neglected diseases. In line with the “One Health” concept it would be of high interest to break the chain between the parasite reservoir in animals and infections in humans reversing the economic losses and sustaining the social well-being in Europe and endemic countries. Thus, new drugs for neglected diseases are still urgently needed.
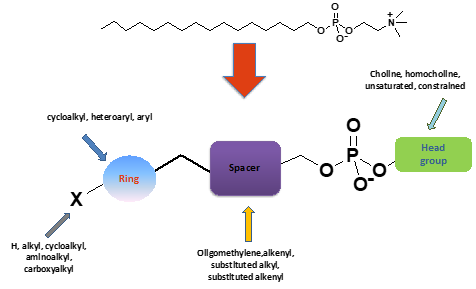
Miltefosine (hexadecylphosphocholine) an ether phospholipid analogue (alkylphosphocholine) is currently the only oral drug available for the treatment of visceral (VL) and cutaneous leishmaniasis (CL). However, miltefosine has several side effects. Aiming at overcoming the side effects of Miltefosine, we embarked on the design and synthesis of constrained ether phospholipid derivatives through the introduction of rings in the lipid portion of alkylphosphocholines and modifications in the head group. These resulted in increased potency against L. infantum intracellular amastigotes accompanied by reduced toxicity against THP-1 macrophages. This line of research was first reported by the group leader, nationally and internationally.
In the context of the FP7-funded project NMTrypI (http://fp7-nmtrypi.eu) this strategy was further optimized through focused lead optimization efforts and resulted in a new Drug Lead with very promising oral antileishmanial activity in mice, hamsters and dogs and extremely low toxicity (https://www.youtube.com/watch?v=9w5V-flKZPU&feature=youtu.be) (https://cordis.europa.eu/project/id/603240/reporting). In addition, it was effective orally in vivo against Τ. brucei acute model of infection and against the long lasting chronic infection model.
Related publications (2000 to date)
- G. E. Magoulas, P. Afroudakis, K. Georgikopoulou, M. Roussaki, C. Borsari, T. Fotopoulou, N. Santarem, E. Barrias, P. Tejera Nevado, J. Hachenberg, E. Bifeld, B. Ellinger, M. Kuzikov, I. Fragiadaki, E. Scoulica, J. Clos, S. Gul, M. P. Costi, W. de Souza, K. C. Prousis, A. Cordeiro da Silva and T. Calogeropoulou* “Design, Synthesis and Antiparasitic Evaluation of Click Phospholipids” Molecules 2021, 26 (14), 4204; https://doi.org/10.3390/molecules26144204
- E. Chazapi, G. E. Magoulas, K. C. Prousis, T. Calogeropoulou* “Phospholipid Analogues as Chemotherapeutic Agents Against Trypanosomatids” Curr. Pharm. Des. 2021. 27, 1790-1806.
https://doi.org/10.2174/1381612826666201210115340 - E. Barrias, L. C. Reignault, T. Calogeropoulou, W. de Souza; “In vitro activities of adamantylidene-substituted alkylphosphocholine TCAN26 against Trypanosoma cruzi: Antiproliferative and ultrastructural effects” Exp. Parasitol. 2019, 206, 107730.
- C. B. Moraes, G. Witt, M. Kuzikov, B. Ellinger, T. Calogeropoulou, K. C. Prousis, S. Mangani, F. Di Pisa, G. Landi, L. D. Iacono, C. Pozzi, L. H. Freitas-Junior, B. S. Pascoalino, C. P. Bertolacini, B. Behrens, O. Keminer, J. Leu, M. Wolf, J. Reinshagen, A. Cordeiro-da-Silva, N. Santarem, A. Venturelli, S. Wrigley, D. Karunakaran, B. Kebede, I. Pöhner, W. Müller, J. Panecka-Hofman, R. C. Wade, M. Fenske, J. Clos, J. M. Alunda, M. Jesús Corral, E. Uliassi, M. L. Bolognesi, P. Linciano, A. Quotadamo, S. Ferrari, M. Santucci, C. Borsari, M.P. Costi, and S. Gul; “Accelerating Drug Discovery Efforts for Trypanosomatidic Infections Using an Integrated Transnational Academic Drug Discovery Platform” SLAS Discovery 2019, 24, 346-361.
- Sant’Anna V., Railbolt M., Oliveira-Menezes A., Calogeropoulou T., Pinheiro J., de Souza W. “Ultraestructural study of effects of alkylphospholipid analogs against nematodes” Exp. Parasitol. 2018, 187, 49-58.
- Fragiadaki, I., Katogiritis, A., Calogeropoulou, T., Brückner, H,, Scoulica “Synergistic combination of alkylphosphocholines with peptaibols in targeting Leishmania infantum in vitro” Int J Parasitol Drugs Drug Resist 2018.
doi. 10.1016/j.ijpddr.2018.03.005 - Borba-Santos, L.M., Ishida, K., Calogeropoulou, T., de Souza, W., Rozental, S. “Adamantylidene-substituted alkylphosphocholine TCAN26 is more active against Sporothrix schenckii than miltefosine” Mem. Inst. Oswaldo Cruz 2016, 111(8) Rio de Janeiro Aug. 2016 Epub July 11, 2016.
- Benítez, D., Medeiros, A., Fiestas, L., Panozzo-Zenere, E.A., Maiwald, F., Prousis, K.C., Roussaki, M., Calogeropoulou, T., Detsi,A., Jaeger, T., Šarlauskas, J., Peterlin Mašič, L., Kunick, C., Labadie, G.R., Flohé,L., Comini, M.A. “Identification of Novel Chemical Scaffolds Inhibiting Trypanothione Synthetase from Pathogenic Trypanosomatids” PLoS Negl Trop Dis 2016, 10(4):e0004617.
- Vila, T. V. M., Ishida, K., de Souza, W., Prousis, K., Calogeropoulou, T., Rozental, S. “Effect of alkylphospholipids on Candida albicans biofilm formation and maturation” J. Antimicrob. Chemother. 2013, 68, 113-125.
- Godinho, J., Georgikopoulou, K., Calogeropoulou, T., de Souza, W., Rodrigues, J. “A novel phosphocholine dinitroaniline hybrid molecule exhibits biological activity in vitro against Leishmania amazonensis.” Experimental Parasitology 2013, 135, 153–165.
- Papanastasiou I., Prousis, K.C, Georgikopoulou, K., Pavlidis, T., Scoulica, E., Kolocouris, N., Calogeropoulou, T.* “Design and synthesis of new adamantyl-substituted antileishmanial ether phospholipids” Bioorg. Med. Chem. Lett. 2010 20 (18), 5484-5487.
- Calogeropoulou, T.;* Angelou, P.; Detsi, A.; Fragiadaki, I.; Scoulica, E. “Design and synthesis of potent antileishmanial cycloalkylidene-substituted ether phospholipid derivatives” J. Med. Chem. 2008, 51, 897-908.
- Karanikolopoulos, N. ; Pitsikalis, M. ; Hadjichristidis, N. ; Georgikopoulou, K. ; Calogeropoulou, T. ; Dunlap, J.R. “pH-Responsive Aggregates from Double Hydrophilic Block Copolymers Carrying Zwitterionic Groups. Encapsulation of Antiparasitic Compounds for the Treatment of Leishmaniasis.” Langmuir 2007, 23(8), 4214-24.
- Kapou, A.; Benetis, N.P.; Avlonitis, N.; Calogeropoulou, T.; Koufaki, M.; Scoulica, E.;. Nikolaropoulos, S.S.; Mavromoustakos, T. “3D-Quantitative Structure-Activity Relationships of New Synthetic Antileishmanial Ring-Substituted Ether Phospholipids” Bioorg. Med. Chem. 2007, 15(3), 1252-65.
- Papazafiri, P; Avlonitis, N.; Angelou, P.; Calogeropoulou, T.;* Koufaki, M.; Scoulica, E.; Fragiadaki, I. “Structure-activity relationships of antineoplastic ring-substituted ether phospholipid derivatives” Cancer Chemother. Pharmacol. 2005, 56, 261-270.
- Avlonitis, N.; Lekka, E.; Detsi, A.; Koufaki, M.; Calogeropoulou, T.;* Skoulica, E.; Siapi, E.; Kyrikou, I.; Mavromoustakos, T.; Tsotinis, A.; Golic Grdadolnik, S.; Makriyannis, A. "Antileishmanial Ring-Substituted Ether Phospholipids" J. Med. Chem. 2003, 46, 755-767.
Related chapters in books
- Theodora Calogeropoulou,* George E. Magoulas, Ina Pöhner, Rebecca C. Wade,2 Joanna Panecka-Hofman, Pasquale Linciano, Stefania Ferrari, Maria Paola Costi,* Nuno Santarem, Ma Dolores Jiménez-Antón, Ana Isabel Olías-Molero, Anabela Cordeiro da Silva and José María Alunda, “Hits and Lead Discovery in the Identification of New Drugs against the Trypanosomatidic Infections” Chapter 10 in: Medicinal Chemistry of Neglected and Tropical Diseases: Advances in the Design and Synthesis of Antimicrobial Agents, (Edited by: Venkatesan Jayaprakash, Daniele Castagnolo, Yusuf Özkay) CRC Press (2019) Pages. 185- 231
- de Souza, W., Godinho, J., Barrias, E., Roussaki, M., Fernandes Rodrigues, J. C. F., Calogeropoulou, T., “Effects of Phospholipid Analogues on Trypanosomatids.” Chapter 13 in Molecular Biology of Kinetoplastid Parasites (Edited by: Hemanta K. Majumder). Caister Academic Press, U.K. (2018) Pages: 221-242. DOI.org/10.21775/9781910190715.13
Patents
- PCTEP2021075969, 22/09/2021 “Compound for use in the treatment of protozoal diseases and process for production of said compound”. Applicant: QuadrEL Srl. Inventors: Calogeropoulou, T. Prousis, K.C., Roussaki, M., Magoulas, G.E., Fotopoulou T.
- GR 20200100577, 22/09/2020 “Compound for use in the treatment of protozoal diseases and process for production of said compound”. Applicant: National Hellenic Research Foundation. Inventors: Calogeropoulou, T. Prousis, K.C., Roussaki, M., Magoulas, G.E., Fotopoulou T.
- US 8,097,752 (issued 17-1-2012) "Antiprotozoal ring-substituted phospholipids" Inventors: T. Calogeropoulou, M. Koufaki, N. Avlonitis, A. Makriyannis