From classical dendrimer to amphiphilic dendrimer
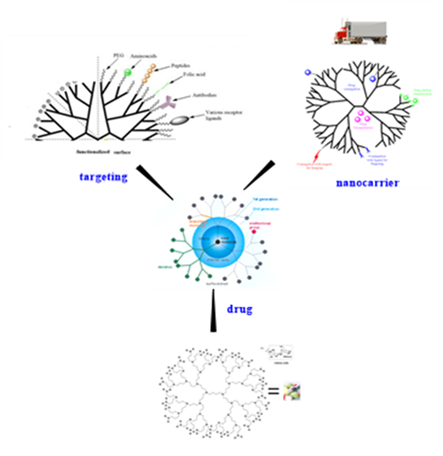
Classical dendrimers are globular unimolecular species containing a core, multiple branching points and a single type of end group constituting their peripheral functionality. They are highly branched and well-defined polymers with precise characteristics resembling small vesicles. Their development in the fields of nanotechnology and materials sciences is considered a milestone in the engineering of macromolecules with a nanometric size (1-10 nm) allowing for passive targeting effects, that reduce the nonspecific toxicity of the drugs carried. Consequently, dendrimers have found a place as nanocarriers in the biomedical development of drug, gene or imaging delivery systems, as well as in tissue targeting, tumor therapy and diagnostics. Some of them are drugs per se.
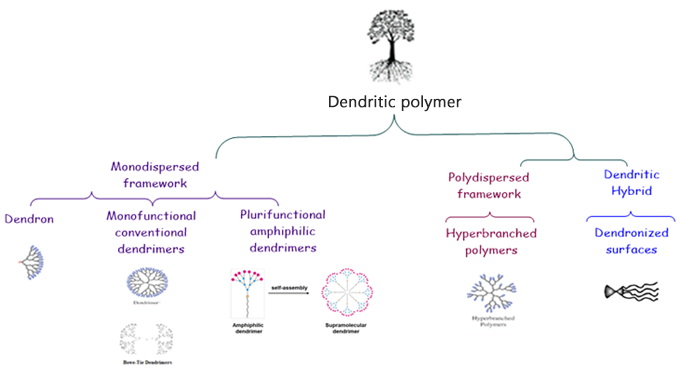
Nevertheless, classical dendrimers are presenting several limitations; complex synthesis, organism’s rapid systemic clearance, significant toxicological issues depending on dendrimer generation, charges, and fragment accumulation, poor drug loading or difficulty to achieve a controlled drug release.
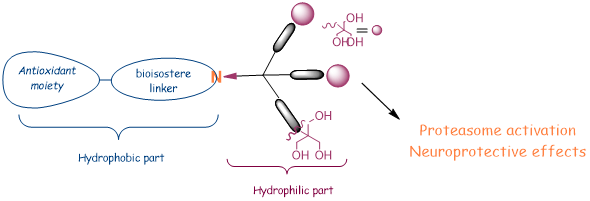
Therefore, the last decade, attention has been paid to smallest biocompatible dendritic structures (e.g dendrons, amphiphilic dendrimers) that present the advantage of moieties multivalence targeted presentation, and are more easily manageable and synthetically accessible.
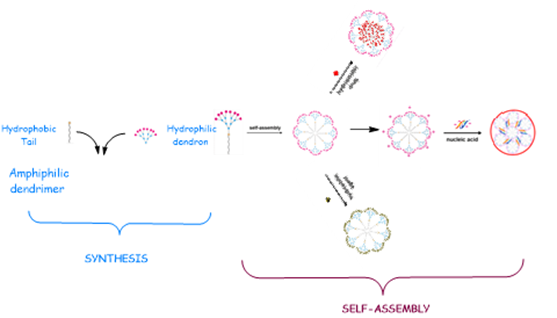
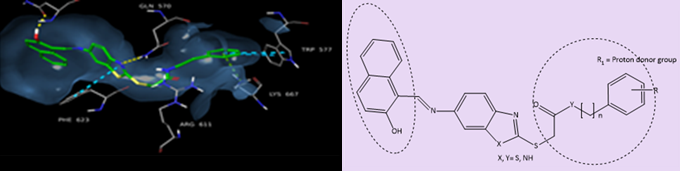
Dendrons because they are molecules containing a functionalized branch with targeted end groups, and a focal point for possible conjugation with bioactive moieties, resulting in the formation of branched hybrid macromolecules whose biological activity is potentially enhanced by targeted multivalent presentation favoring supramolecular dynamics of interaction with complex biological systems.
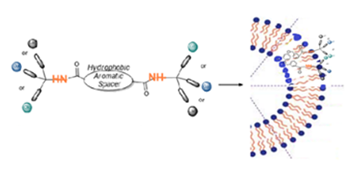
Amphiphilic dendrimers because they are branched bi-functionalized molecules possessing self-assembly properties that allow the formation of largest non-covalent supramolecular structures mimicking globular covalent dendrimers, or because they can become components of other artificial complex highest nanosystems (e.g., liposomes, cubosomes, polymersomes, nanoemulsions, microemulsion) that with a plethora of applications in the neutra/pharmaceutical industry.
From classical to biocompatible glycodendrimers
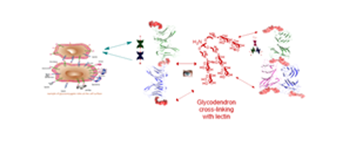
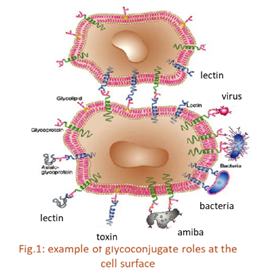 Carbohydrates play a vital role through their interactions with lectins, which are specific carbohydrate binding proteins prevalent in living organisms implicated in intercellular recognition events such as cell adhesion and migration, cell differentiation and apoptosis, and infection by viral, parasitic and bacterial pathogens.
Carbohydrates play a vital role through their interactions with lectins, which are specific carbohydrate binding proteins prevalent in living organisms implicated in intercellular recognition events such as cell adhesion and migration, cell differentiation and apoptosis, and infection by viral, parasitic and bacterial pathogens.
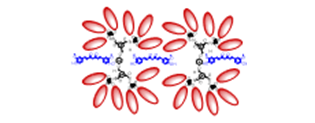
Lectins are usually aggregated into oligomeric structures which can also dissociate into dimers or monomers on whose surface a binding pocket is located. This provides a suitable arrangement for high affinity multivalent binding to suitable carbohydrate analogs.
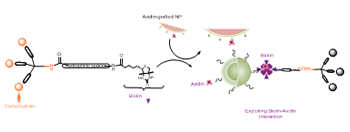
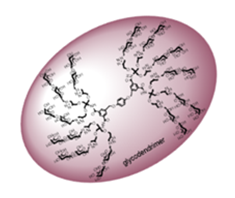 Current interest in the development of potential pharmaceuticals based on carbohydrate-lectin interactions has led to the synthesis of glycoconjugate compounds such as glycodendrimers with a multivalent carbohydrate periphery that confers a capacity of binding to biological macromolecules such as lectins via multiple and simultaneous non-covalent H-bonding interactions. These cooperative interactions confer high binding affinity and specificity to the carbohydrate-ligand complexes typically required to mediate the essential biological processes mentioned above and such compounds are foreseen to have a wide range of potential applications such as anti-adhesion drugs, drug delivery systems, functional antigens or anti-tumor vaccines.
Current interest in the development of potential pharmaceuticals based on carbohydrate-lectin interactions has led to the synthesis of glycoconjugate compounds such as glycodendrimers with a multivalent carbohydrate periphery that confers a capacity of binding to biological macromolecules such as lectins via multiple and simultaneous non-covalent H-bonding interactions. These cooperative interactions confer high binding affinity and specificity to the carbohydrate-ligand complexes typically required to mediate the essential biological processes mentioned above and such compounds are foreseen to have a wide range of potential applications such as anti-adhesion drugs, drug delivery systems, functional antigens or anti-tumor vaccines.


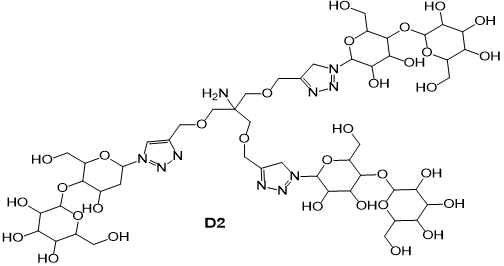
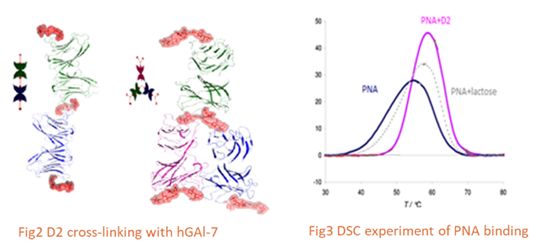
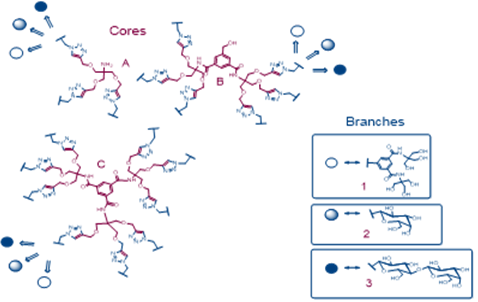
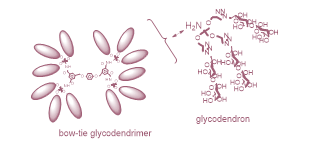
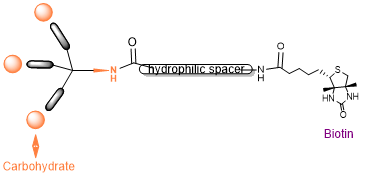
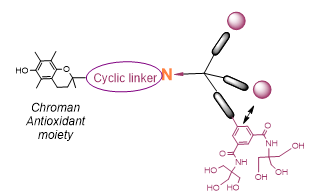
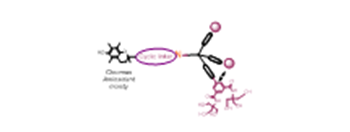
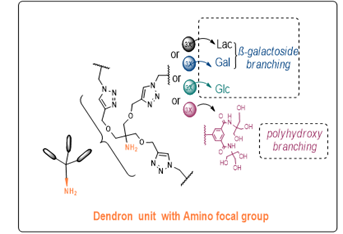 Based on promising physicochemical and crystallographic results obtained from in vitro studies with lectin [1,2], various glycerol- and glycodendrons, containing the schematically described focal amino group, are used as versatile precursors for the construction of small libraries of molecules used in various areas of interest as:
Based on promising physicochemical and crystallographic results obtained from in vitro studies with lectin [1,2], various glycerol- and glycodendrons, containing the schematically described focal amino group, are used as versatile precursors for the construction of small libraries of molecules used in various areas of interest as: